Homosexual orientation by choice
This study, led by Alan Sanders at Chicago's North Shore University HealthSystem Research Institute, directly compared about 2,000 homosexual and heterosexual men in order to find genetic differences between them.
They didn't find much. The researchers picked up some signal near several genes that are expressed in the brain. They also detected some genetic differences near the gene for the thyroid stimulating hormone receptor.
This gene plays a role in Graves' disease, a thyroid autoimmune disorder that, according to a recent Danish study, occurs more often in gay men. These and other related findings, write Sanders and his colleagues, "point to a possible connection between thyroid function and sexual orientation in men."
What can we take away from these modest results? The study, as the authors recognize, is fairly small as far as modern genetic studies go, and so its power to find genes linked to same-sex differences is limited.
The results are also limited to men—the genetics of sexual orientation in women, or of transgender individuals is likely to be different. There are no definitive findings here, only clues for later research to build on.
THE STUDY ITSELF BELOW
Family and twin studies suggest that genes play a role in male sexual orientation. We conducted a genome-wide association study (GWAS) of male sexual orientation on a primarily European ancestry sample of 1,077 homosexual men and 1,231 heterosexual men using Affymetrix single nucleotide polymorphism (SNP) arrays. We identified several SNPs with p < 10−5, including regions of multiple supporting SNPs on chromosomes 13 (minimum p = 7.5 × 10−7) and 14 (p = 4.7 × 10−7). The genes nearest to these peaks have functions plausibly relevant to the development of sexual orientation.
On chromosome 13, SLITRK6 is a neurodevelopmental gene mostly expressed in the diencephalon, which contains a region previously reported as differing in size in men by sexual orientation. On chromosome 14, TSHR genetic variants in intron 1 could conceivably help explain past findings relating familial atypical thyroid function and male homosexuality.
Furthermore, skewed X chromosome inactivation has been found in the thyroid condition, Graves’ disease, as well as in mothers of homosexual men. On pericentromeric chromosome 8 within our previously reported linkage peak, we found support (p = 4.1 × 10−3) for a SNP association previously reported (rs77013977, p = 7.1 × 10−8), with the combined analysis yielding p = 6.7 × 10−9, i.e., a genome-wide significant association.
Introduction
While the usual combination of sex chromosomes (XX or XY) predicts sexual orientation and behavior for the vast majority of humans (as heterosexual), variation exists: a stable minority of men (3~4%) are homosexual1 and male sexual orientation appears to be bimodally distributed with most men rating themselves as predominantly heterosexual (Kinsey scale 0–1) or homosexual (Kinsey scale 5–6)1,2,3,4,5. Male sexual orientation is moderately heritable (30~40%), but is multifactorial, with evidence of multiple genetic and environmental contributions, via family studies6,7,8,9,10,11, twin studies4,12,13,14,15,16, and segregation analyses8,10,11,17. Although focused (i.e., chromosome X) and genome-wide linkage studies (GWLS) of affected (i.e., concordant) sibling pairs have been applied to the trait8,18,19,20,21,22, these have been in relatively small samples prior to our own GWLS23, which showed genome-wide significant linkage to the pericentromeric region of chromosome 8 and strong support for linkage to the previously reported Xq28 region. Although genetic variant(s) contributing to development of male sexual orientation have been mapped to pericentromeric chromosome 8 and chromosome Xq28, the linkage peaks are large and specific trait genes have not been identified.
Genetic association studies for male sexual orientation have been sparse, small, and used either a convenience sample with proxy markers (e.g., blood type) or usually a candidate gene approach, and have yielded negative findings (strongest finding being a nominal p = 0.03)24,25,26,27. No genome-wide association studies (GWAS) for male sexual orientation have been heretofore published in the peer-reviewed literature. One meeting report (2012 ASHG) from the company 23andMe analyzed male sexual orientation (N = 13,733 European ancestry men) as a continuous variable28 and identified its strongest association (p = 7.1 × 10−8, direction unstated) in rs7701397729, an intronic SNP in NKAIN3(Na+/K+ transporting ATPase interacting 3), which lies in a multipoint linkage peak on the pericentromeric region of chromosome 8 identified by our lab23. To extend our gene mapping efforts for the trait, we report here the results from analyzing 1,109 homosexual and 1,231 heterosexual primarily European ancestry men in the first published GWAS on the trait.
Results/Discussion
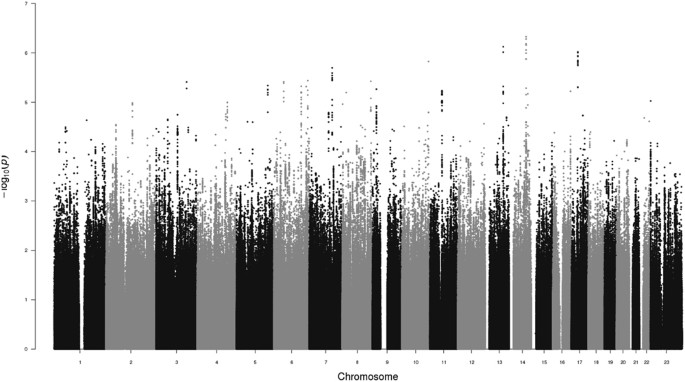
We detected several promising regions of multiple SNPs in the 10−5–10−7p-value range, as seen in the Manhattan plot (Fig. 1), though no SNP reached genome-wide significance (5 × 10−8). The most prominent of these regions were on chromosomes 13 (minimum p = 7.5 × 10−7, rs9547443) and 14 (p = 4.7 × 10−7, rs1035144), where some SNPs had 10−7 < p < 10−6 (each region with 9 to 10 SNPs with p < 10−5, Table S1). There are a number of genes of relevance to the trait in and around these regions, which we describe below. We further note that the most significant SNP (rs77013977, p = 7.1 × 10−8) in the 23andMe male GWAS29 was nominally associated (p = 4.1 × 10−3) in our own GWAS. We used a meta-analytic statistic that did not need direction of effect, Fisher’s combined probability test30, which yielded p = 6.7 × 10−9 for this SNP, which is the first reported genome-wide significant association for the trait. As previously noted29, rs77013977 is an intronic SNP in NKAIN3, which is one of a family of four proteins (NKAIN1–4) suggested to be critical for neuronal function31.
The strongest associated region on chromosome 13 (rs9547443, p = 7.5 × 10−7) was located between SLITRK6 (SLIT and NTRK like family member 6, ~60 kb centromeric to region) and SLITRK5 (~1.8 Mb telomeric), with SLITRK1 located ~2.0 Mb centromeric. Members of the SLITRK protein family are brain-expressed neuronal transmembrane proteins that regulate neuronal outgrowth, survival, and synapse formation; SLITRKs have significant homology to the secreted axonal growth-controlling SLIT family of proteins and also homology to the neurotrophic tyrosine kinase receptor (NTRK) family32,33,34. SLITRK6 is expressed especially in the diencephalon (which contains a region previously reported as differing in size in men by sexual orientation35), and SLITRK1 and SLITRK5 have their highest expression in the cerebral cortex32,33,34. Gene families, such as the SLITRK family that are important for neurodevelopment and are implicated as candidate genes for various neuropsychiatric phenotypes34, are also of potential relevance to behavioral phenotypes such as sexual orientation.
On chromosome 14, TSHR (thyroid stimulating hormone receptor) spans the region around our most significant SNP (rs1035144, p = 4.7 × 10−7), and includes a cluster of SNPs with association p < 10−5 in intron 1. TSHRencodes a G protein-coupled transmembrane receptor for thyrothropin (thyroid stimulating hormone) and thyrostimulin, manifests some constitutive activity (i.e., ligand independent), and is a major controller of thyroid cell metabolism36,37,38. While the main tissue of interest and expression for TSHR is the thyroid gland, TSHR is expressed in other tissues including brain especially in neuron-rich areas (e.g., hippocampus)39. TSHR codes for the major autoantigen in the autoimmune hyperthyroidism of Graves’ disease, which is associated (p < 10−20 with OR’s 1.4~1.5) with intron 1 polymorphisms40,41,42,43,44,45,46,47,48.
A recent population-based study found that 5,351 same-sex married men among the assayed population of 2,252,751 Danish men had an elevated rate ratio of Graves’ disease (RR = 1.88; 95% CI = 1.08–3.01), a finding which held when excluding men with HIV/AIDS49. The authors49 speculate on the possibility that a genetic (or other prenatal) factor might tie together this increased risk for a type of hyperthyroidism (Graves’ disease) with separate observations of lower body weight for homosexual versus heterosexual men (independent of diet or exercise)50,51,52. Females with Graves’ disease have been reported to manifest biased X chromosome inactivation53,54,55, and skewed X chromosome inactivation has also been reported in mothers of homosexual men compared to age-matched mothers of heterosexual men56.
Furthermore, a recent retrospective chart review of 790 adolescents (8 to 17 years) previously admitted to a child psychiatry service found 15 mothers with a history of thyroid dysfunction during pregnancy, 16 adolescents with a history of same-sex attraction and/or gender nonconformity, and 12 overlapping mother-offspring pairs with both (p < 0.0001), suggestive of a possible relationship57. Thus converging findings, including suggestive evidence from the current study, point to a possible connection between thyroid function and sexual orientation in men.
The main limitations of the current study include an exclusive focus on males, sampling primarily from one ancestral group (European), combination of two datasets, and most notably the modest sample size for a GWAS on a trait with complex genetics. Additional and larger sample sizes would be required to assess which loci might breach genome-wide significance for association in a single study, and to increase the number of such loci (as typically is the case with phenotypes with manifesting complex genetics58,59).
Nevertheless, our study provides support for the top finding from a previous meeting report of a GWAS on the trait29, reaching genome-wide significance for the combined analysis of rs77013977 (p = 6.7 × 10−9) on pericentromeric chromosome 8. In addition, the current study’s top two association peaks (p < 10−5; Fig. 1) provide interesting and perhaps trait-relevant examples of their closest genes on chromosomes 13 (SLITRK6) and 14 (TSHR), though these potential connections are best characterized as speculative. The continued genetic study of male sexual orientation should help open a gateway to other studies focusing on genetic and environmental mechanisms of sexual orientation and development. Detectable genetic variants predisposing to homosexuality would have alternative alleles, which would necessarily predispose to heterosexuality, thus contributing to understanding of both typical heterosexual and minority homosexual orientations.